| Items |
 /Asset/2103-MW-UR.jpg /Asset/2103-MW-UR.jpg 2101-MW-UR D70 Cured Durometer Hardness Medical Wearable Adhesive |
 /Asset/2101-MW-UR.jpg /Asset/2101-MW-UR.jpg 2103-MW-UR D77 Cured Durometer Hardness Medical Wearable Adhesive |
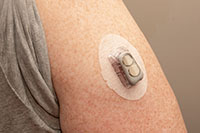 /Asset/2022-MW.jpg /Asset/2022-MW.jpg 2022-MW D60 Cured Durometer Hardness Medical Wearable Adhesive |
|||
| Description |
N/A
Adhesives for medical wearable device assembly are formulated without IBOA (isobornyl acrylate), a known skin irritant and TPO, a material of concern. The 2000-MW series of products cures with UV/LED light to seal, encapsulate, coat, and bond components in medical wearables such as drug delivery devices. The materials pass ISO 10993-10 / -23 for sensitization and irritation and ISO 10993-5 for cytotoxicity and bond to PC, PVC, SS, PI, TPU and other difficult-to-bond-to substrates.
|
|||||
| Brands | N/A Dymax® | |||||
| Nominal Viscosity at 20 Revolutions Per Minute (rpm) Speed | N/A 5,500 cP | N/A 5,500 cP | N/A 700 cP | |||
| Cured Durometer Hardness | N/A D77 | N/A D70 | N/A D60 | |||
| Water Absorption (25 Degree Celsius (ºC) Temperature, 24 Hours (h)) | N/A 2.1 % | N/A 0.1 % | N/A | |||
| Uncured Appearance | N/A Colorless to light yellow transparent liquid | N/A Colorless to light yellow transparent liquid | N/A | |||
| Tensile at Break | N/A 24.8 MPa3,600 psi | N/A | N/A | |||
| Cured Elongation at Break | N/A 80 % | N/A | N/A 8 % | |||
| Modulus of Elasticity | N/A 1,020.4 MPa148,000 psi | N/A | N/A | |||
| Boiling Water Absorption (24 Hours) | N/A 4.5 % | N/A | N/A | |||
| Fluoresces | N/A Yes | N/A | N/A | |||
| Recommended Substrates | N/A Acrylonitrile Butadiene Styrene (ABS) Polycarbonate (PC) Polycyclohexylene Dimethylene Terephthalate Glycol (PCTG) Polyethylene Terephthalate Glycol (PETG) Polyvinyl chloride (PVC) Thermoplastic Polyurethane (TPU) | N/A Acrylonitrile Butadiene Styrene (ABS) Polycarbonate (PC) Polyvinyl chloride (PVC) Stainles Steel (SS) Thermoplastic Polyurethane (TPU) | N/A Acrylonitrile Butadiene Styrene (ABS) Aluminum Glass Polycarbonate (PC) Polyethylene Terephthalate Glycol (PETG) Polymide (PI) Polyurethane (PU) Stainless Steel (SS) Styrene-Acrylonitrile (SAN) | |||
| Industry Standards | N/A International Organization for Standardization (ISO 10993-10) International Organization for Standardization (ISO 10993-23) International Organization for Standardization (ISO 10993-5) | N/A International Organization for Standardization (ISO 10993-10) International Organization for Standardization (ISO 10993-5) | N/A | |||
| Features |
N/A
|
N/A
|
N/A
|
|||
| Additional Information |
N/A
LED-Curable Medical Wearable Adhesive For Skin Sensitivity Concerns The 2101-MW-UR is blazing the trail for the next generation in advanced medical-grade adhesives for wearables. As part of the 2000-MW product line, these adhesives are specifically designed to address skin sensitivity concerns through the removal of IBOA (isobornyl acrylate). 2101-MW-UR adhesive passes ISO 10993-10 skin irritation and sensitization and ISO 10993-5 cytotoxicity testing, making it an ideal option for smart medical wearable devices. 2101-MW-UR is a light-cure adhesive that bonds using LED or ultra-violet light with a medium viscosity. The material also uses Ultra-Red® fluorescing for simple in-line inspection of UV curing materials. The medical adhesive forms a highly reliable bond to many plastics such as ABS, PC, PCTG, PETG, PVC, and TPU. This makes it an ideal substance to use for the assembly of medical smart devices, patient monitoring devices, large-volume injectors, or diabetes care devices. |
N/A
Moisture-Resistant Adhesive for Medical Wearables with Ultra-Red® Fluorescing Dymax® 2103-MW-UR is designed for rapid assembly of medical wearable devices where skin sensitivity is a concern by eliminating the use of H317 monomers which may cause sensitization (including IBOA). 2103-MW-UR meets ISO 10993-5 Cytotoxicity, ISO 10993-23 Intracutaneous Irritation, and ISO 10993-10 Sensitization standards. The material is intended for applications using plastics and metals and can be cured with broad-spectrum, 365 nm, 385 nm, or 405 nm LED light. Formulated with Ultra-Red® fluorescing technology, 2103-MW-UR fluoresces bright red under low-intensity black light (365 nm). The bright red fluorescence contrasts extremely well on plastics that naturally fluoresce blue in color, such as PVC, and greatly assists with visual inspection of the bond-line area. Ideal applications include medical smart devices, hearable devices, wearable injectors, diabetes care monitors, vital sign monitoring devices, sleep monitoring devices, and pain management devices. Dymax® medical device adhesives contain no nonreactive solvents and cure upon exposure to light. Their ability to cure in seconds enables faster processing, greater output, and lower processing costs. |
N/A
Low Water Absorption Encapsulant, Coating and Bonding Adhesive for Wearable Medical Devices Dymax® 2022-MW is a versatile general bonding adhesive, coating, and encapsulant with low water absorption and minimized water ingression. It offers extremely low water absorption of 0.5% making it ideal for the assembly or encapsulation of electronic wearable medical device components. Formulated without IBOA, a known skin irritant, and TPO, a material of concern, 2022-MW is compliant with ISO 10993-5 for cytotoxicity standards. This classification makes it an ideal material for wearable medical devices where proximity to the skin for extended periods of time is a concern. 2022-MW is recommended for use as a coating or encapsulant on RFID chips or sensors housing assemblies and single- or multi-use medical devices where moisture ingression is a concern. It bonds well to stainless steel, aluminum, glass, and printed circuit boards. This material has no solvents added and cures in seconds upon exposure to broad-spectrum UV or 365 nm LED light. |
|||
|
|
||||||